COVID-19 mRNA Vaccines Registrational Trials
Abstract:
Early investigations into mRNA vaccines for SARS-CoV-2 showed promise, with Pfizer-BioNTech and Moderna reporting a 95% reduction in symptomatic COVID-19. These vaccines bypassed the typical four-year testing period, sparking concerns about safety. Trials focused on mild symptoms and excluded key demographics like children and pregnant women. Monitoring for severe adverse events was lacking, leading to potential underestimation of risks.The data suggests a significant number of vaccinations are needed to prevent a single case of COVID-19, prompting calls for transparent discussions about their benefits and risks.
The analysis of Pfizer’s COVID-19 vaccine trial by Michels et al. raises several concerns. Unblinded placebo recipients who later received the vaccine were merged with the vaccine group, potentially biasing results. Deaths among vaccinated subjects increased notably after week 20, with delays in reporting, particularly for cardiac-related deaths,more frequent in the vaccinated group. These issues weren’t addressed in the trial’s original paper or Pfizer’s safety report. Had they been disclosed, the FDA might have reconsidered the vaccine’s authorization, potentially prolonging the process. Recent animal studies suggest mRNA vaccines could induce cardiomyopathy, adding to concerns. Additionally, an abrupt halt in COVID-19 cases among vaccinated individuals after day 12 raises doubts about the results’ validity.
The representation of immediate immunity post day 12 following BNT162b2 injection suggests unexpected rapid decline in infection rates compared to the placebo group, raising questions about its reliability. Contradictory data on neutralizing antibody titers also challenges the notion of immediate immunity, casting doubt on the vaccine’s efficacy. Registrational trials weren’t designed to address infection prevention, and since 2021, it’s recognized that mRNA COVID-19 vaccines offer limited protection against transmission.Studies indicate reduced disease severity but fail to thoroughly investigate transmission risk. Comprehensive evidence from cohort studies, notably within the Cleveland Clinic Health System, underscores the limitations of mRNA vaccines in preventing COVID-19 despite booster administration.
The Cleveland Clinic study comparing COVID-19 incidence among “up-to-date” and “not up-to-date” individuals challenges the efficacy of mRNA vaccines. The plot, featuring a Simon-Makuch hazard analysis, reveals no significant difference in cumulative incidence between the two groups after the XBB Omicron variant became dominant.With vaccine efficacy questioned, two narratives emerge: one emphasising protection against severe outcomes, and the other advocating for vaccination in combination with natural immunity to enhance protection.
Early investigations on mRNA vaccinations indicated the potential of these biologicals for preventing SARS-CoV-2 infection. Based on the first randomized controlled trials sponsored by Pfizer-BioNTech (New York, U.S.; Mainz, Germany) and Moderna Inc. (Massachusetts, U.S.), researches concluded that there was a remarkable 95% relative risk reduction of symptomatic COVID-19.
Prior to the rapid authorization process, no vaccines had been permitted for market release without undergoing a testing period of at least four years, the record set by Merch & Co., Inc (New Jersey, U.S.) in 1967 with the development of the world’s first mumps vaccine. Pfizer’s vaccine (BNT162b2) completed the process in seven months. The previously established 10-15-year time-frame for clinical evaluation of vaccines was deemed necessary to ensure adequate time for monitoring the development of serious health diseases such as cancers and autoimmune disorders. Historical accounts bear witness to instances where vaccines were prematurely introduced to the market under immense pressure, only to reveal disabling or even fatal serious health diseases later on. Against this backdrop, it is not surprising that so many medical and public health experts voiced concerns about the COVID-19 mRNA vaccines bypassing the normal safety testing process.
Vaccines were viewed as the hope for ending the crisis during early phases of the outbreak. However, scrutiny of the registration trial data has revealed some concerning findings. The trials were not designed to evaluate whether mRNA vaccines can effectively reduce the risk of severe illness or death. They failed to demonstrate the efficacy of mRNA vaccines in lowering severe COVID-19 cases, hospitalizations, or fatalities. Despite claims of high efficacy, the primary focus of the trials was on alleviating mild COVID-19 symptoms.
Additionally, certain crucial demographics such as children, pregnant women, the elderly, and those with underlying health issues were excluded from the trials. There are deficiencies in monitoring severe adverse events, potentially leading to an underestimation of the vaccine’s safety. Data on absolute risk reduction suggests that a considerable number of individuals require vaccination to prevent a single case of COVID-19, a benefit that may be offset by potential risks. Therefore, there is a critical need for transparent discussion on the potential benefits and risks of COVID-19 mRNA vaccines.
Analysis of Pfizer trial’s weekly mortality over 33 weeks
This representation of the Pfizer trial by Michels et al. showcases the weekly count of subject deaths from July 27, 2020 to March 13, 2021. Solid bars denote BNT162b2 recipients, grey bars signify the placebo group, and hatched bars represent previously unblinded placebo subjects who later received BNT162b2. The solid line represents the cumulative death count for the BNT162b2 group and the dotted line for the placebo group.
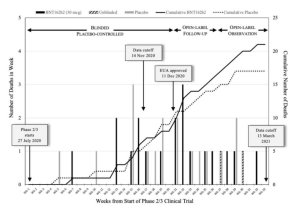
Image Source: Michels et al., 2023
The analysis highlights several concerning aspects of Pfizer’s COVID-19 vaccine trial. Initially, unblinded placebo recipients later receiving the vaccine were combined with the vaccine group, potentially skewing the results. The trial was divided into 3 periods, with deaths among vaccinated subjects increasing notably after week 20. Moreover, there were delays in reporting deaths, particularly cardiac-related ones, which were more frequent in the vaccinated group. These discrepancies weren’t addressed in the trial’s original paper or Pfizer’s safety report. The FDA might have reevaluated the vaccine had these issues been disclosed, potentially prolonging the authorization process. Recent animal studies suggest mRNA vaccines could induce cardiomyopathy. Additionally, the trial data showed an abrupt halt in COVID-19 cases among vaccinated individuals after day 12, contradicting biological plausibility and raising doubts about the validity of the results.
Charts illustrating Pfizer trial irregularities in reporting of COVID-19 cases and humoral immune responses (antibody titers)
This representation indicates an unusual pattern post day 12 following the BNT162b2 injection. While the placebo group continued experiencing cases, the BNT162b2 group showed a sudden decline in infection rates, suggesting unexpected immediate immunity.

Image Source: Palmer M, et al., 2023
The charts illustrated neutralizing antibody titers on the day of the first injection (D1) through subsequent days reveals a gradual elevation of neutralizing antibodies against SAR-CoV following mRNA inoculation. This contradicts the expectation of rapid attainment of complete clinical immunity. By day 21 post-first dose, neutralizing antibody levels exhibited only slight elevation, reaching their peak on day 28, a time-point well beyond the typical administration of the second dose. This inconsistency between clinical outcomes and antibody response data raises doubts about the graphical representation of immediate immunity on day 12, calling into question its reliability.
The ability to halt or significantly reduce infection is generally considered crucial for vaccine efficacy. However, the registrational trials by Pfizer and Moderna were not designed to address this issue. Instead, their focus was on mitigating COVID-19 symptoms. Since 2021, it has been recognized within the scientific community that mRNA COVID-19 vaccines do not offer full protection against either transmission or infection. Even experts supported by the vaccine industry have acknowledged a maximum reduction in transmission of 61% in 2021. The Omicron subvariants have been linked to a 30-50% decrease in transmission post-booster administration. However, this benefit is incremental and temporary, with protection against Omicron infection lasting only a few months. Despite higher antibody titers against SARS-CoV-1 post-vaccination, these levels decline faster in mRNA recipients compared to those with natural infection. The impact of reduced disease severity among vaccinated individuals on the risk of transmitting the virus to others has not been thoroughly investigated in controlled clinical trials.
The most compelling evidence highlighting the failure of the COVID-19 mRNA vaccine’s ability to provide protection against COVID-19 originates from two extensive cohort studies involving employees within the Cleveland Clinic Health System (CCHS) after the bivalent mRNA boosters became available.
Cleveland Clinic study showing increasing COVID-19 cases with increasing mRNA vaccinations
Cleveland Clinic study illustrates COVID-19 incidence among participants based on the number of prior mRNA vaccine doses received. The study shows rising case rates associated with increased COVID-19 mRNA vaccine doses.

Image Source: Shrestha et al., 2023
The figure displays the results of the previous study, showcasing the cumulative incidence of COVID-19 among study participants segmented by the number of prior mRNA vaccine doses administered. Day 0 corresponds to September 12, 2022, marking the introduction of the bivalent vaccines to CCHS employees. It is evident from the data that case rates escalated in correlation with an increasing number of mRNA injections.
Cleveland Clinic study showing increased COVID-19 cases for subjects most “up to date” with mRNA vaccinations
Cleveland Clinic study comparing cumulative COVID-19 incidence between “up-to-date” and “not up-to-date” individuals based on CDC-defined vaccination status. The plot includes point estimates and 95% confidence intervals along the x-axis.
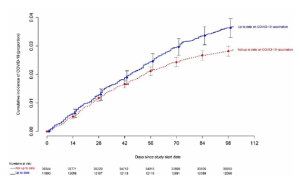
Image Source: Shrestha et al., 2023
The figure presents another unexpected discovery, this time from the second Cleveland Clinic study, featuring a Simon-Makuch hazard plot comparing the cumulative COVID-19 incidence in the “up-to-date” and “not up-to-date” with respect to CDC-defined vaccination status. Day 0 corresponds to January 29, 2023, the day the XBB lineages of the Omicron variant became dominant in Ohio. Both charts display point estimates and 95% confidence intervals along the x-axis.
With the efficacy of the product now firmly in question, the vaccination campaign has adopted two narratives to justify the ongoing administration of COVID-19 vaccines. The first narrative suggests that although COVID-19 mRNA products may not prevent infections entirely, they still offer protection against severe disease, hospitalization, and mortality. The second narrative argues that the protection associated with the mRNA inoculation, when combined with natural infection, surpasses that of natural infection alone, thereby advocating for the importance of vaccination.
Compiled by: Vincci Ng
Supervised by: George Ngui
Reference Link:
Olliaro, P. (2021, February 17). What does 95% COVID-19 vaccine efficacy really mean? THE LANCET Infectious Disease. https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(21)00075-X/fulltext
COVID-19 mRNA Vaccines: Lessons Learned from the Registrational Trials and Global Vaccination Campaign. (2024, January). ResearchGate. https://www.researchgate.net/publication/377667508_COVID-19_mRNA_Vaccines_Lessons_Learned_from_the_Registrational_Trials_and_Global_Vaccination_Campaign
